|
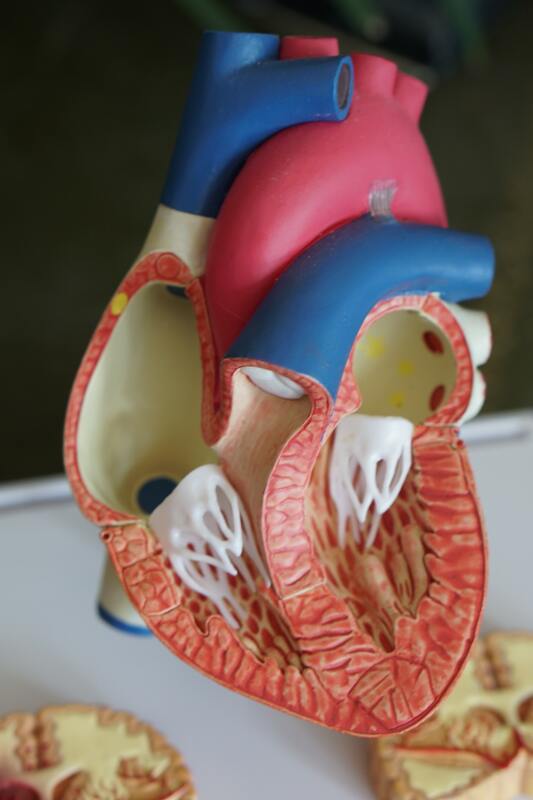
CLEVELAND, OHIO-S4 Medical, a medical device company focused on protecting the esophagus from damage during catheter ablation procedures for atrial fibrillation (AF), announces that the US Food and Drug Administration (FDA) has approved its Investigatory Device Exemption (IDE) for a human clinical study of the ESOLUTION esophageal catheter. The study is a prospective, multi-center, randomized controlled, single-blinded clinical study to assess a reduction of esophageal lesions attributable to catheter ablation treatment of AF.
Dr. Raul Weiss, MD, FACC, Professor of Clinical Medicine and Director of the Electrophysiology Fellowship Program at The Ohio State University Medical Center, will serve as principal investigator of the study. "This is an interesting study as it will provide the first FDA randomized study of a device for protecting the esophagus from injury by ablation energy. This device also uses vacuum suction to hold the esophagus and then moves the entire segment, solving the issue with the trailing edge. Finally, the device is designed to provide for controllable degrees of bidirectional deflection," says Dr. Weiss. The chief medical officer and co-inventor of the device, Emile Daoud, MD, Electrophysiology Section Chief at The Ohio State University, is equally enthusiastic: "It has taken some time, but we have developed a simple but clever way to safely and successfully deviate the esophagus. If successful, this will be well received by electrophysiologists as an added benefit for managing patients undergoing AF ablation." Since its founding in 2017, S4 has achieved several exciting milestones towards bringing the ESOLUTION device to market, including a successful first in a human study, an issued patent, and now this IDE approval. "We are delighted the FDA has granted this IDE and excited to kick off this study and evaluate the ESOLUTION device in a larger study. This IDE is proof of the excellent progress we've made at S4," says co-founder and CEO William Fuller. About S4: S4 Medical Corp. is a medical device company focused on innovative solutions for cardiac procedures. The company's primary focus is a simple, yet comprehensive solution for reducing complications to the esophagus during catheter ablation treatment for atrial fibrillation. S4's team is motivated by providing advanced solutions for superior healthcare. For more information, visit S4 at www.S4medical.com on LinkedIn and Twitter.
0 Comments
Leave a Reply. |
Archives
March 2023
|